Although characteristic of Carlin-type deposits, invisible Au in pyrite is also observed in epithermal (low- and high-sulfidation) and porphyry deposits. Some famous examples include Yanacocha (Peru), Pueblo Viejo (Dominican Republic), Brucejack (Canada), Lihir (Papua New Guinea), and Dexing (China). Perhaps most interestingly… invisible Au has also been found in the epithermal system at the active Kawah Ijen Volcano (Indonesia).
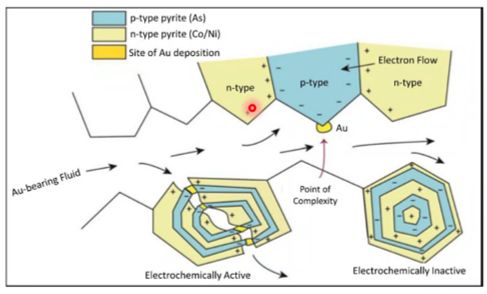
What’s the mechanism for the precipitation of invisible Au in pyrite? Even if Au-bearing phases are undersaturated in condensed liquids, they can adsorb onto the surface of growing pyrite crystals. Adsorption of Au is facilitated by the update of As, which causes pyrite crystals to develop p-type semiconductive properties (Prokhorov and Lu, 1971; Mironov et al., 1981; Starling et al., 1989; Möllerand Kerten, 1994).
The negatively charged species (e.g., Au(HS)-) is attracted to the positively charged pyrite surfaces and the metals are subsequently incorporated in pyrite. This can occur either by electrochemical reduction of the positively charged metal in the species to form native metal or nanoparticles or, perhaps, through the coupled substitution of Cu+, Ag+, and Au+ with As3+ for Fe2+. On the other hand, neutral species in could be in more random contact with the pyrite surfaces, but, once there, the incorporation mechanisms are the same (Williams-Jones et al., 2009).
Invisible Gold at Kawah Ijen Volcano (Java, Indonesia)

At Kawah Ijen Volcano (Java, Indonesia) there is a crater that is ~1km in diameter that contains a hyperacidic crater lake and at least one actively degassing fumarole mound along the lake shore (it is possible there is additional degassing under the lake). In addition to the fumarole mound, the lake shore also contains multiple centers of high-sulfidation epithermal alteration.
Scher et al. (2013) collected gas condensate, gas, mineral precipitate, and rock chemistry data, the sum of which allowed the authors to conclude that in addition to altering the rocks at Kawah Ijen, the condensed vapors also served as the agent for metal transport (concentrations of Cu and As in the condensed fumarolic vapors sampled at the active fumarole mound reached 3 ppm and 3.8 ppm, respectively). Additionally, the authors concluded that Au was transported as a hydrated bisulfide or chloride gas species and condensed about 250m below the floor of the pre-1817 crater. Given the extreme acidity of the condensed liquid, the Au metal speciation in this fluid is likely to have been dominated by either a negatively charged or neutral species, e.g., AuCl2–or AuHS (Stefánsson and Seward, 2003, 2004; Williams-Jones et al., 2009).
Although Au-, Ag-, and Cu-bearing phases were undersaturated in the condensed vapors, these metals were able to concentrate in the rocks (in the alunite-pyrite zone) through adsorption as negatively charged or neutral species onto the surfaces of growing pyrite crystals, which had acquired p-type semiconductive character due to incorporation of As. They metals were incorporated in the pyrite either by their electrochemical reduction to form native metal nanoparticles or through coupled substitutions with As for Fe and S, or in the case of Cu, through direct exchange for Fe. The positive correlation of Cu, Ag, and Au concentrations in pyrite with that of As are explained by both these mechanisms (Scher et al., 2013).
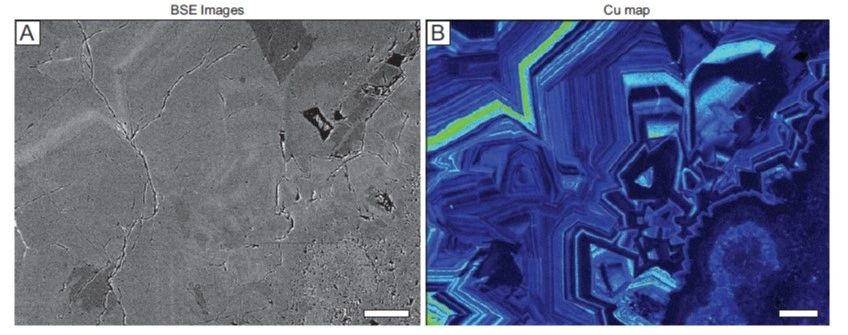
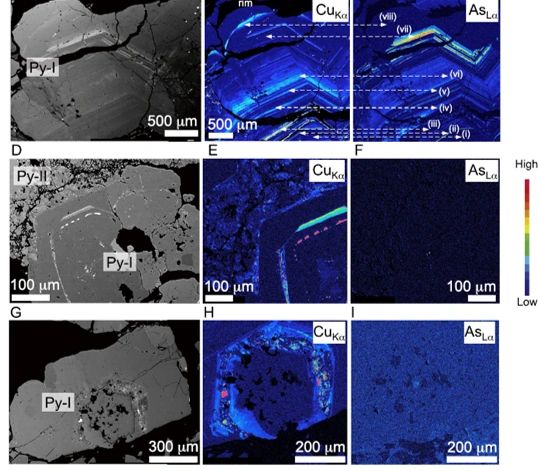
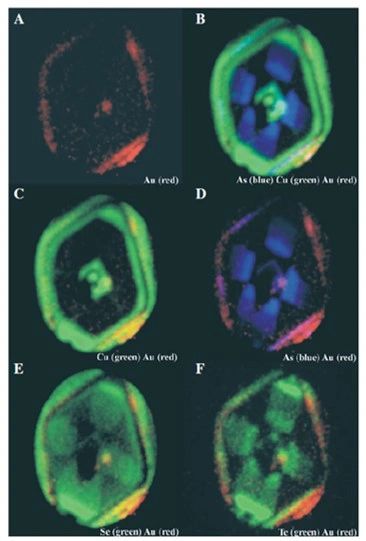
What Does Invisible Gold Look Like?
Look and enjoy!