Fig. 1. Montmorillonite spectra celebrating Spectalucation July. The y-axis is reflectance (%) and the x-axis is wavelength (nm).
Our blog posts thus far have concentrated more on geochemistry and general themes so we thought for July… let’s get into some spectral topics to break up either the extreme heat that we’re feeling at our home office or the more mild temperatures that our friends in the southern hemisphere are feeling. Welcome to Spectralucation July!
For an introduction to what is spectroscopy and how does knowledge of it affect my daily life, let’s start with the age-old question: “why are plants green?”
In order to answer this question, we first need to define some basic spectroscopy concepts:
- Spectroscopy – the study of the absorption and emission of electromagnetic (EM) radiation (e.g., light) by matter; measured as a function of wavelength.
- Reflectance – the measure of the proportion of light (or other radiation) striking a surface which is reflected.
- Absorption – absorption of EM radiation is how matter (typically electrons bound in atoms) takes up a photon’s energy and transforms EM energy into internal energy of the absorber. This absorbed energy affects fundamental molecular dynamics and the state of energy and is the sum of electronic energy, vibrational energy, and rotational energy (the latter occurs in non-solids).
When light interacts with a surface, it can be absorbed (and possibly transmitted), partly scattered, and reflected.
Photosynthesis is the process of the plant converting atmospheric gas carbon dioxide and water into simple sugars, producing oxygen as a by-product. To do this, the plant requires energy and it gets that energy from the light that it absorbs.
By absorbing light, the chlorophyll molecules in the leaf also absorb some of the energy carried by the light. Their preference is to use a combination of red and some blue light depending on the cell type. The unused green light is reflected from the leaf. We see this reflected light, which is what makes the leaves appear green (Fig. 2).
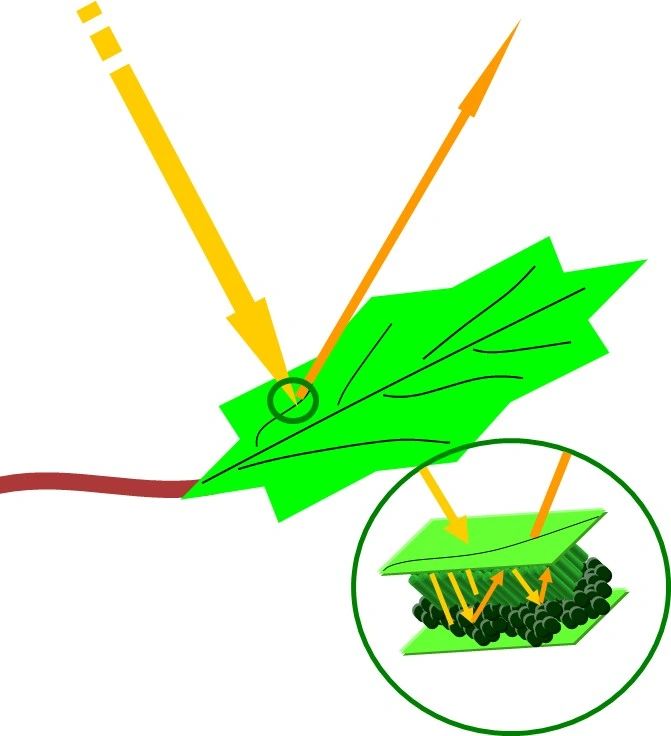
Tying these concepts back to the identification of minerals…
The principals of reflectance spectroscopy for mineral analysis are identical to the way in which we detect or view colors (visible RGB light, ~400-700nm) in objects around us. Incident energy interacts with a material (in the above example, a leaf, but for us geologists, a rock), some of the infrared (IR) energy (VNIR-SWIR is ~400-2500nm) is absorbed by the material and the wavelengths that are reflected results in diagnostic spectral signatures for minerals, like the montmorillonite spectra that we see in Fig. 1.