To close out our series on geometallurgy, we are presenting an overview on some highly complex topics that contain a lot of underlying thermodynamics and kinetics (we are unfortunately not going to get super deep into this here), both of which are the natural, best friends of geochemists.
What is extractive metallurgy?
Extractive metallurgy, unlike mineral processing, generally aims at extracting and purifying specific elements from ores or mineral concentrates. While the link between primary ore characteristics and mineral processing outcomes is important, the ore may undergo changes as it is processed, which can affect subsequent extraction processes.
Therefore, a complete understanding of the entire value chain from primary ore characteristics to the refined product must be well integrated and understood.
There are three broad categories of metallurgy – hydrometallurgy, pyrometallurgy, and electrometallurgy:
- In hydrometallurgy the process of leaching raw materials can be classified into four broad categories: in-situ leaching, percolation leaching, tank/vat leaching, and agitated leaching. In all cases, the pregnant (i.e., metal-containing) solution is treated further through processes, such as solvent extraction and selective precipitation, to recover the valuable metals.
- In pyrometallurgy chemical changes in ores and concentrates occur at high temperatures, ranging from 100 to 3000°C. Various processes are applied, depending on the products required.
- In electrometallurgy electrical energy is used to produce metals by electrolysis. It is typically the last stage in metal production.
Herein are highly summarized descriptions of metal concentration, extraction, and refinement that will hopefully get you started in your journey of understanding these fascinating (and very chemistry-rich!) topics.
The steps:
Ore concentration is the first step in our recovery process and involves the removal of gangue particles. This can be achieved using gravity separation, froth floatation, magnetic separation, and leaching processes.
This process is followed by metal extraction processes or recovery, which includes roasting, calcination, reduction, and electrolytic reduction.
Finally, the impure metal produced during the process of reduction is refined in order to become pure metal.
The steps by which a flowsheet is developed is dependent on the reactivity of the metal (e.g., Fig. 1).
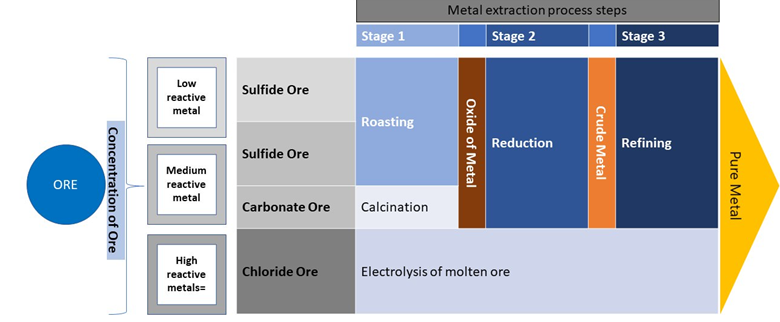
Metal Concentration
Below are examples of common methodologies of metal concentration:
- Gravity separation: generally used for the concentration of oxides and carbonate ores and used when gangue particles are lighter than the ore. This method involves the passing of crushed ore particles through a stream of water during which the lighter gangue particles are washed away.
- Froth floatation: used to remove gangue particles from sulfide ores. In this method, the crushed ore and water are added to a tank in which a frother, such as pine oil, is added and then air is blown under pressure to create the froth. The gangue particles will be wetted by water and the metal rises with the froth.
- Magnetic separation: used when either metal or gangue particles have magnetic properties. In this method, the crushed ore is passed over conveyor belts with magnetic rollers.
- Leaching: used to concentrate ores, including Al, Ag, and Au. In this method, the crushed ore is treated with a solvent that dissolves the ore leaving behind impurities.
Metal Extraction Processes (Recovery)
Below are common examples of metal extraction processes, or recovery:
- Roasting: heating of concentrated metal ore in presence of air. This is mainly done for metal sulfide ores to convert them into their respective oxides.
- Calcination: process by which ore is heated in the absence or in a limited supply of air. This method is commonly used to convert metal carbonates and metal hydroxides into their respective oxides.
- Reduction and Electrolytic Reduction: metal oxides produced in the roasting and the calcination processes further undergo reduction to produce impure metal.
- Reduction can be done using an appropriate reducing agent such as carbon or hydrogen. The reduction of metal oxide can also be achieved by using a displacement reaction in which a more reactive metal replaces the less reactive one.
- Electrolytic reduction is used for highly reactive metals, such as sodium and magnesium, which cannot be obtained by the reduction of their respective metal oxides since these metals have a high affinity for oxygen. Therefore, these metals are isolated by the electrolytic reduction of their molten salts.
Metal Refinement
Below is a common refinement method:
- One of the refining methods is electrolytic refining by which a strip of impure metal is made at the anode while pure metal is made at the cathode. The metal salt solution is used as the electrolyte in this process. When an electric current is passed through the cell, the pure metal from the anode goes into the electrolyte and the same amount of pure metal then is deposited at the cathode. During this process, the insoluble impurities settle below the anode as anode mud.
Tying it all together
Prior geomet blog posts, including ore texture, deportment, and commentary on mineralogy are all relevant even at this advanced stage of the value chain.
For example, in hydrometallurgy:
- Porosity (i.e., texture) influences the breakage behavior of the ore, and hence the particle size distribution of the furnace feed.
- The presence of hydrous minerals or carbonates causes volatile loss on heating. Where their modal abundance is significant, treatment must be undertaken prior to any furnace process.
- Leaching processes are affected by many of the same primary ore characteristics as mineral processing operations (see Table 1). Of particular importance are mineral grain sizes and associations, ore and gangue mineralogy and mineral abundances, as well as mineral liberation in the crushed material.
On the other hand, for pyrometallurgy:
- Mineralogy and microfabric no longer play a role in these latter processes, although the primary ore will influence the chemical composition of the pregnant solution.
- The solid leach residues, by contrast, are essentially chemically modified primary ores.
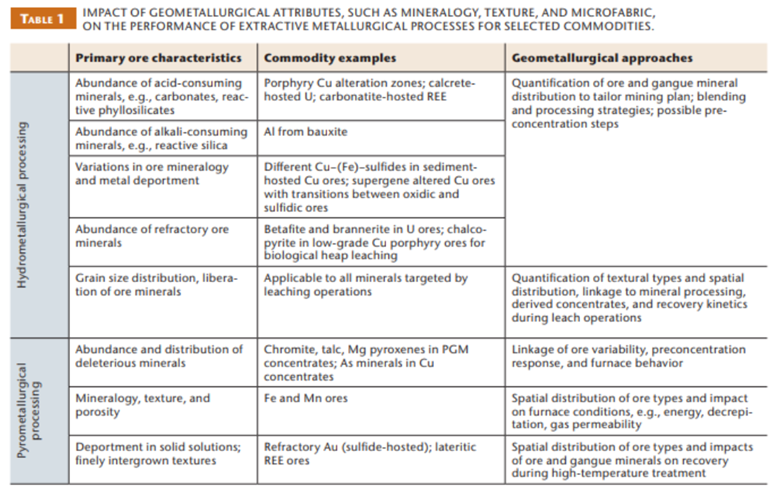
Check out this table from Chetty et al. (2023) for more examples:
References
- Chetty, D., Nwaila, G.T., Xakalashe, B., 2023. Fire and Water: Geometallurgy and Extractive Metallurgy, Elements, 19, 365-370.
- Olvera, O. (2018, May 22-25). Metal Extraction and Recovery [Short Course]. Geometallurgy Practical Short Course, University of British Columbia, Vancouver, BC, Canada.
- FlexBooks 2.0, 2024, Extraction of Metals from Ores, accessed 13 January 2024, <https://flexbooks.ck12.org/cbook/cbse-chemistry-class-10/section/3.7/primary/lesson/extraction-of-metals-from-their-ores/>.